Women younger than 50 years old should especially be aware of the rare risk of blood clots with low platelets after vaccination. If you received a JJJanssen COVID-19 Vaccine here is what you need to know.
Covid 19 Vaccine Janssen News Articles Etc European Pharmaceutical Review
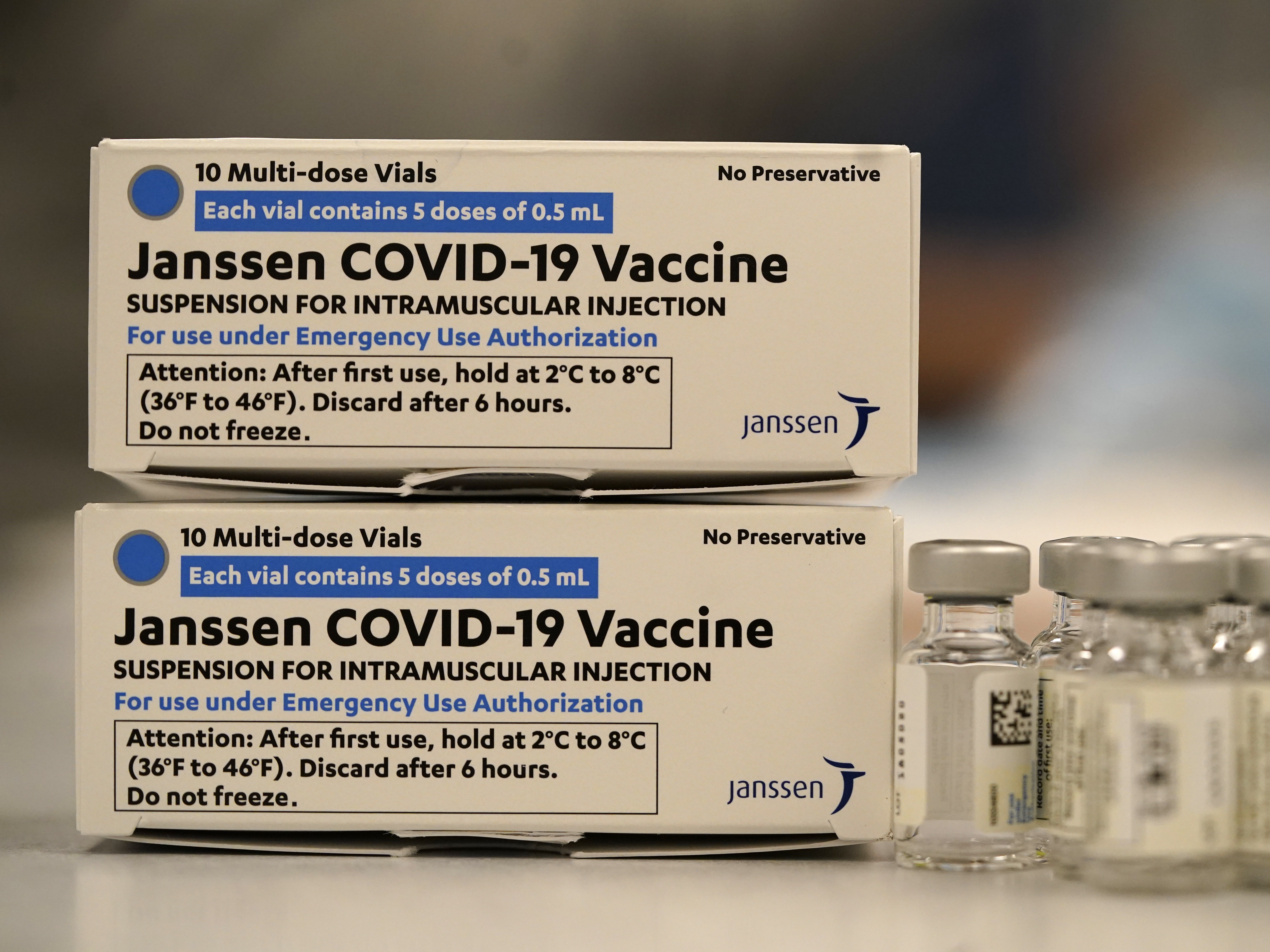
The US Food and Drug Administration FDA has granted Janssens single-shot COVID-19 vaccine Emergency Use Authorization EUA.
Covid vaccine janssen fda. Janssen COVID-19 Vaccine Johnson Johnson. Today the FDA is announcing revisions to the vaccine recipient and vaccination provider fact sheets for the Johnson Johnson Janssen COVID-19 Vaccine to. FDA Flickr cc.
FDA approves Comirnaty COVID-19 Vaccine mRNA which was previously known as Pfizer-BioNTech COVID-19. There are other COVID-19 vaccines available for which this risk has not been seen. The Janssen COVID-19 Vaccine is an unapproved vaccine that may prevent C OVID-19.
Johnson Johnsons Janssen JJJanssen COVID-19 Vaccine. Er ist seit dem 11. However women younger than 50 years old especially should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed.
Third doses of Covid-19 vaccines will aid public health and require more contract manufacturing. Effective April 23 2021 CDC and FDA recommend that use of the Janssen COVID-19 Vaccine resume in the United States. Will people who received Johnson Johnsons Janssen JJJanssen COVID-19 Vaccine need a booster shot.
The Janssen COVID-19 Vaccine is an unapproved vaccine. This is the third vaccine to receive such approval in the US and it allows the Janssen COVID-19 Vaccine to be distributed. Coronavirus Updates Moderna submitted data from 344 volunteers who got a third shot of the vaccine.
Es handelt sich um einen Vektor-Impfstoff. Food and Drug Administration FDA recommended use of Johnson Johnsons Janssen JJJanssen COVID-19 Vaccine resume in the United States after a temporary pause. Reports after the use of JJJanssen COVID-19 Vaccine suggested an increased risk of a rare adverse event called thrombosis with thrombocytopenia syndrome TTS.
It is likely that people who received a JJ COVID-19 vaccine will need a booster dose. These people can receive any FDA-authorized vaccine including the Janssen COVID-19 Vaccine. The US Centres for Disease Control and Prevention CDC and Food and Drug Administration FDA have announced a plan to offer the US general public a third shot of either PfizerBioNTechs or Modernas Covid-19 vaccines from 20 September.
There is no FDA -approved vaccine to prevent COVID -19. Moderna Asks FDA To Authorize A Booster Shot Of Its COVID-19 Vaccine. On April 23 CDC and the US.
The Janssen COVID-19 Vaccine vaccination schedule is a single dose. COVID-19 Vaccines FDA COVID-19 Vaccines August 23 2021. The Food and Drug Administration FDA announced that its Vaccines and Related Biological Products Advisory Committee VRBAC will meet on Sep 17 to discuss COVID-19 vaccine third doses and specifically address the Pfizer-BioNTech supplemental Biologics License Application for administration of a third dose of that vaccine.
As stated above as an exception people with a history of an episode of an immune-mediated syndrome characterized by thrombosis and thrombocytopenia such as HIT should be offered another FDA-authorized COVID-19 vaccine ie mRNA vaccine if it has been 90 days or less since their illness. Because the JJJanssen vaccine wasnt given in the United States until 70 days after the first mRNA vaccine doses Pfizer-BioNTech and Moderna the data needed to make this decision arent available. Additionally the FDA has extended the expiration dating for the refrigerated Janssen COVID-19 Vaccine after reviewing information submitted.
Der COVID-19-Impfstoff COVID-19 Vaccine Janssen Ad26COV2S wird von der Firma Janssen-Cilag hergestellt einer Tochterfirma von Johnson Johnson. Mai hat die Ständige Impfkommission STIKO empfohlen den Impfstoff erst bei. The FDA has authorized the emergency use of the Janssen.
The FDA has authorized the emergency use of the Janssen COVID-19 Vaccine to prevent COVID-19 in individuals 18years of age and older under an Emergency Use Authorization EUA. Johnson Johnsons COVID-19 Vaccine The Companys COVID-19 vaccine leverages the AdVac vaccine platform a unique and proprietary technology that was also used to develop and manufacture Janssens European Commission-approved Ebola vaccine regimen and construct its investigational Zika RSV and HIV vaccines. In an ongoing clinical trial 21895 individuals 18 years of age and.
März 2021 in Deutschland für die Impfung von Personen ab 18 Jahren zugelassen. HAS THE JANSSEN COVID-19 VACCINE BEEN USED BEFORE. On April 13 2021 the FDA and CDC recommended a pause in the use of Janssen COVID-19 Vaccine due to reports of serious adverse events of.
Fda Extends Shelf Life Of Johnson Johnson Covid 19 Vaccine To Six Months

Fda Clears Third Covid 19 Vaccine From Janssen Daic

Fda Adds J J Covid 19 Vaccine Warning Cidrap

Texas Recommends Resuming Johnson Johnson Vaccine Shots After Fda S Green Light Abc13 Houston

U S Authorizes Another Batch Of Johnson Johnson S Covid 19 Vaccine
Fda Some J J Vaccine Is Good To Go But Some Isn T 2021 06 11 Bioworld

Ohio State Pauses Johnson Johnson Vaccine Appointments Amid Fda And Cdc Precautionary Guidance
Fda Warning About J J Vaccine Creates More Challenges For Fighting Covid Crisis

Arc Pauses Johnson Johnson Covid 19 Vaccine Per Cdc And Fda Recommendations Austin Regional Clinic
Risk Benefit Analysis Of The Fda S Decision To Pause J J Vaccine Blog Ivt
Fda Halts Covid 19 Vaccine Production At Emergent Facility
Johnson Johnson Asks Fda To Authorize Its Covid 19 Vaccine For Emergency Use Los Angeles Times

Fda Advises Pausing J J Covid Vaccine After Blood Clotting Issue Affects 6 Women Kills 1

Cdc Fda Joint Statement Janssen Johnson Johnson Covid 19 Vaccine Sweetwater Hospital Association
Johnson Johnson Covid Vaccine Analysts Are Cautiously Optimistic

Fda Says Single Dose Shot From J J Prevents Severe Covid 19 Chicago News Wttw
Cdc Fda To Review J J Shot After 6 Blood Clot Cases Reported Out Of Nearly 7m Doses Wjct News

Coronavirus Eu Rejects Some Johnson Johnson Covid Vaccines Over Contamination News Dw 11 06 2021
تويتر Janssen Global على تويتر New Data Show Our Single Shot Covid 19 Vaccine Generated Persistent Activity Against The Rapidly Spreading Delta Variant Amp Other Highly Prevalent Sars Cov 2 Viral Variants Https T Co 1m1nv1wdg3 Janssen S Vaccine